Treatments
THE MOST COMMON TREATMENTS FOR NON MUSCLE-INVASIVE BLADDER CANCER (NMIBC)
The type of treatment for Non-Muscle Invasive bladder cancer (sometimes called early or superficial bladder cancer) depends very much upon the cell type, grade and stage of the cancer. The main aim of treatment is to remove the growth as early as possible and prevent the cancer returning and progressing in the future.
There are three main types of treatment:
- Transurethral Resection of Bladder Tumour (TURBT)
- Intravesical therapies, including BCG (intravesical immunotherapy)
- Mitomycin (intravesical chemotherapy)
Treatment for low risk non-muscle invasive
bladder cancer
If you have low risk bladder cancer, the main treatment is TURBT which is surgery to remove the
cancer from the bladder lining. If the cancer is completely removed during
TURBT, you may not need any on-going treatment, although you should receive a single one-off dose of Mitomycin chemotherapy immediately following your TURBT (within 24 hours). This reflects a specific recommendation from the NICE Bladder Cancer Guideline that recommends such treatment for all bladder cancer cases.
Treatment for intermediate risk
non-muscle invasive bladder cancer
If you have intermediate (moderate) risk bladder cancer you should usually
have a 6 week course of chemotherapy
directly into your bladder after the TURBT operation.
Treatment for high risk non-muscle
bladder cancer
If your first TURBT operation shows you have high risk bladder
cancer, you will usually have a second TURBT, ideally within 6 weeks of the
first, to check if any cancer remains and how far the cancer has grown. You may then have:
- A course of BCG vaccine treatment directly into the bladder
- An operation (primary cystectomy) to remove the bladder
Your urologist will talk to you about the risks and benefits of these treatments and the grade and stage of your cancer and the likelihood of it spreading. They will also tell you about effects and implications of both treatments and their impact on your life.
The Patient Choices section offers some guidance on treatment choices.
Read ABC UK's statement on the current BCG supply situation. [link to BCG statement]
FURTHER INFORMATION ABOUT THE TREATMENTS
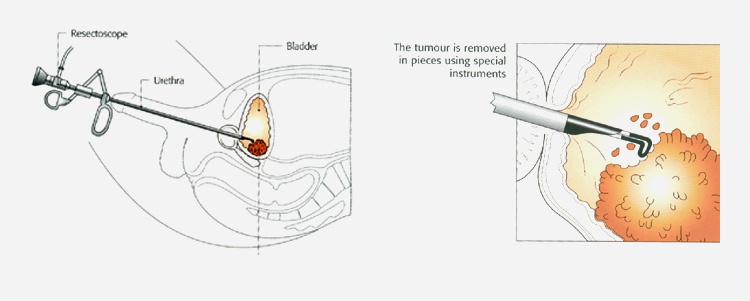
TURBT is the principal treatment for non-muscle invasive bladder cancer. It is the first treatment that most patients will have after receiving a provisional diagnosis. It is an operation to remove the cancerous growth and seal the tissue around where the growth was. You will have either a general or spinal anaesthetic, and a detailed telescopic examination of the inside of the bladder. As this is performed via the urethra (water pipe) no surgical incision is required.
Any abnormal areas within the bladder are carefully assessed by the surgeon and all visible tumour is then removed (resected) with a hot loop (diathermy). Tumour samples are then washed out of the bladder. A catheter is needed postoperatively and most patients will be given a single chemotherapy treatment into the bladder (intravesical chemotherapy) after the operation before leaving hospital, which has been shown to significantly decrease the risks of a bladder tumour reoccurring. The risks of this operation are:
- blood in the urine
- urine infection
- bladder perforation
- requirement for a prolonged (7-10 days) period of catheterisation
- a burning feeling on passing urine (dysuria)
- worse urinary tract symptoms with urine frequency and urgency
The operation is usually performed as a day case or with a one night stay in hospital.
IMPROVEMENTS IN VISUAL DETECTION
Various techniques are currently being evaluated to improve visual detection of bladder cancer, either at diagnosis or at recurrence.
Photodynamic diagnosis (PDD) or Blue Light Cystoscopy
Many hospitals will offer photodynamic diagnosis (PDD) or Blue Light Cystoscopy which enables the surgeon to assess for tumour cells that might not be detected otherwise. This is not dissimilar to a standard cystoscopy and TURBT (where white light is used) but involves a special dye being washed into the bladder one hour before the operation. The ‘dye' is absorbed by cancer cells and glows bright pink under a blue light shone into the bladder during the surgery. The areas of cancer are therefore much easier to see and remove by TURBT. This can also be used when telescope tests are used during follow up.
Narrow Band Imaging (NBI)
Narrow band imaging (NBI)
uses a narrow bandwidth of light (415nm - 540nm), for a similar purpose, and
does not require prior catheterisation or administration of an intravesical
photosensitiser. Trials have been
conflicting in terms of the advantage offered by these treatments, and recommendations
will likely become clearer with future studies.
INTRAVESICAL TREATMENT
There are two main intravesical treatments:
1 Intravesical immunotherapy with BCG
2 Intravesical chemotherapy, usually with a drug called Mitomycin.
Their role and side-effects are quite different and it is worth asking your consultant to make clear which one will be used.
What happens when you have immunotherapy or chemotherapy?
In both cases the treatment will be carried out by an experienced nurse on a weekly basis. This is usually done for 6 weeks on an outpatient basis. Each time you will need to have a small catheter inserted in to your bladder through which the treatment, as a fluid, is introduced. This isn't usually too painful but can be a little uncomfortable. You will need to retain the fluid in your bladder for about an hour or two after which you can empty your bladder in the usual way, with some precautions.
How does BCG work?
BCG (Bacille Calmette Guerin) was originally developed as a tuberculosis vaccine, it is also effective at helping to slow or stop bladder cancers recurring or spreading. It is thought BCG works by stimulating the body's own immune system which then attacks the cancer cells in the bladder. This does not work for every type of cancer and bladder cancer is unusual in responding to immunotherapy treatment and is an area of ongoing research.
How does intravesical chemotherapy work?
Urologists first tried putting chemotherapy drugs (which are normally given by injection into the bloodstream) directly into the bladder. As with BCG, the results were very promising, and patients did not get the side effects normally associated with chemotherapy such as nausea or hair loss. Although a number of drugs have been tried over the years the most commonly used one in the UK is called Mitomycin. Unlike BCG, Mitomycin is not currently given as a ‘top-up' maintenance treatment although this is the subject of ongoing research.
Effects of Treatment
Both BCG and chemotherapy can produce significant effects both within your bladder and more generally. Sometimes, this is bad enough to stop the treatments. See the separate sections on BCG and chemotherapy for more information on treatment effects.
Overall both treatments have been extensively used for many years and are generally safe.
In general, intravesical therapy is only recommended for patients who may be quite likely to recur or progress to a more advanced stage (intermediate or high risk non-muscle invasive bladder cancer). Broadly speaking intravesical therapy reduces your risk of recurrence and so is definitely worthwhile.
BCG OR INTRAVESICAL CHEMOTHERAPY
BCG produces generally better results but has more serious treatment effects. Intravesical chemotherapy has fewer serious treatment effects but may not be as effective as BCG. If you have a high-risk tumour you would usually receive BCG, if an intermediate risk tumour you would often receive intravesical chemotherapy.
When you are given a single dose of intravesical chemotherapy during your operation, it is because when a tumour is removed from the bladder there is a worry that some of the cancer cells can break off and stick to the bladder wall where they can grow and form new tumours. It is now the recommended standard practice to put a single dose of intravesical chemotherapy into the bladder at the end of the surgery which destroys any cancer cells floating about and can reduce the chances of getting further recurrences. BCG is not given at this time because of the risk of absorption of the BCG through the operation site.
Cystectomy is recommended for non-invasive tumours or carcinoma-in-situ that are resistant or refractory to intravesical treatment. While offering long-term control, removing the bladder has major implications and potential complications, and would not be undertaken unless the risks of progression are high.
Radiotherapy and systemic chemotherapy (i.e. into the whole body) are not effective treatments for non-muscle invasive bladder cancer.
Regular surveillance is the cornerstone for the management of non-muscle invasive bladder cancer. It is also essential alongside any intravesical treatment, to ensure that the treatment is effective, and for early control of any recurrence should they develop.
NEW TREATMENTS
New treatments for bladder cancer are under development and may be offered to you. These are normally offered on a trial or research basis. Some of these are listed below:
Device Assisted Intravesical Therapy
New methods of giving chemotherapy are currently being tested. Two of these are:
- EMDA - electromotive drug administration or electrically stimulated intravesical chemotherapy (sometimes also called iontophoresis).
- Hyperthermic intravesical chemotherapy - which involves heating the wall of the bladder at the same time as using mitomycin.
Only certain hospitals, usually with a special interest in bladder cancer, are currently offering these treatments and NICE Guidelines have approved their use but only as part of clinical trials until more evidence on their effectiveness is gathered.
EMDA
This might be suggested if you have a high grade bladder cancer or a cancer which recurs after chemotherapy or BCG treatment. An electrical current is used which helps the bladder lining to absorb the chemotherapy.
Your bladder is emptied, and you are given an ultrasound to check it is empty. A catheter (tube) with a small electrode is put into your bladder. Your bladder is washed out with sterile water. Patches containing electrodes are put on to your skin, on the lower abdomen, and these are attached to a small, box-like, generator. The chemotherapy drug is put into your bladder through the catheter. The generator is switched on and sends a small current through the electrodes and the current draws the drug into the cells of the bladder lining. It may tingle, but is not painful and lasts about half an hour, when the bladder is drained and the catheter removed. The treatment is usually given weekly for 6 weeks.
Possible treatment effects are:
- Redness or itching from the patches
- Passing urine more frequently
- Pain on passing urine
- Blood in urine
- Urine infection
- Tiredness or flu like symptoms or fever
Hyperthermic Mitomycin C
This may be suggested as an outpatient treatment before or after surgery to remove non-muscle invasive bladder cancer - sometimes referred to as early or superficial bladder cancer. It can be called hyperthermic mitomycin C, the Synergo technique or intravesical microwave hyperthermia with chemotherapy.
You are given a local anaesthetic into your bladder and a catheter put into the bladder through the urethra. The chemotherapy drug and a probe are put into the bladder through the catheter. The probe heats up the bladder lining by microwaves - this appears to make cancer cells more sensitive to the chemotherapy.
NICE has given approval for this treatment to be used to treat bladder cancer tumours that have not grown further than the bladder lining and has worked well for those with high grade, non-muscle invasive bladder cancer where BCG treatment has not worked. There are hopes that this treatment will help avoid bladder removal in these patients. It is only currently available as part of a clinical trial.
Possible treatment effects: you may feel an irritation of the bladder or symptoms of cystitis, although these can pass quickly.
HIVEC - Hyperthermic Intra-VEsical Chemotherapy
This is another way of giving mitomycin C where a device heats the mitomycin C and the drug is passed through a catheter into the bladder. This is being tested in the HIVEC II clinical trial.
New intravesical regimens are also under development, for example looking at combination regimens of chemotherapy and immunotherapy.
New intravesical agents are being trialed such as gemcitabine or PDL-1 antibodies.
How we help you
Latest News / Events
Setting up a support group for your patients - Webinar for Nurses - Mon 18 Aug 2025 08/07/2025
This is a FREE ABC UK online Zoom session, which is being run in response to requests from urology and cancer nurses, on how to set up and run a successful patient support group. The session will be led by one of the ABC UK Patient Support team, together with two advanced nurse specialists...











